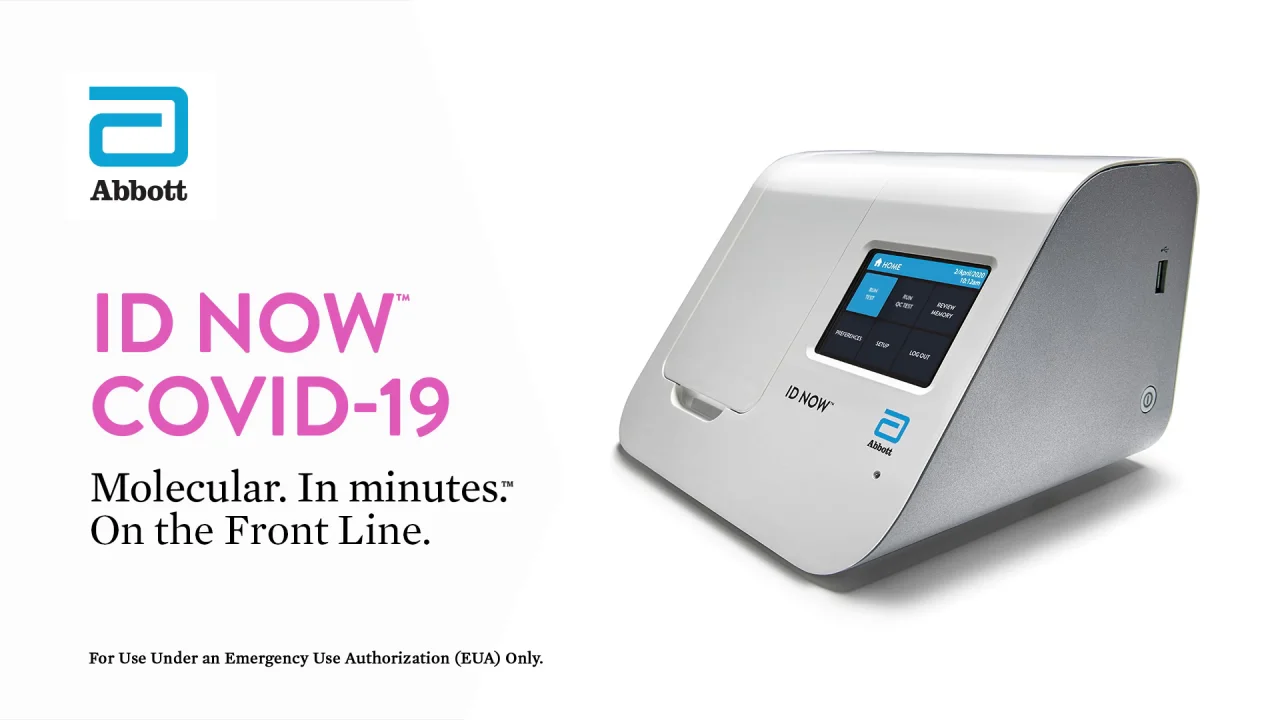
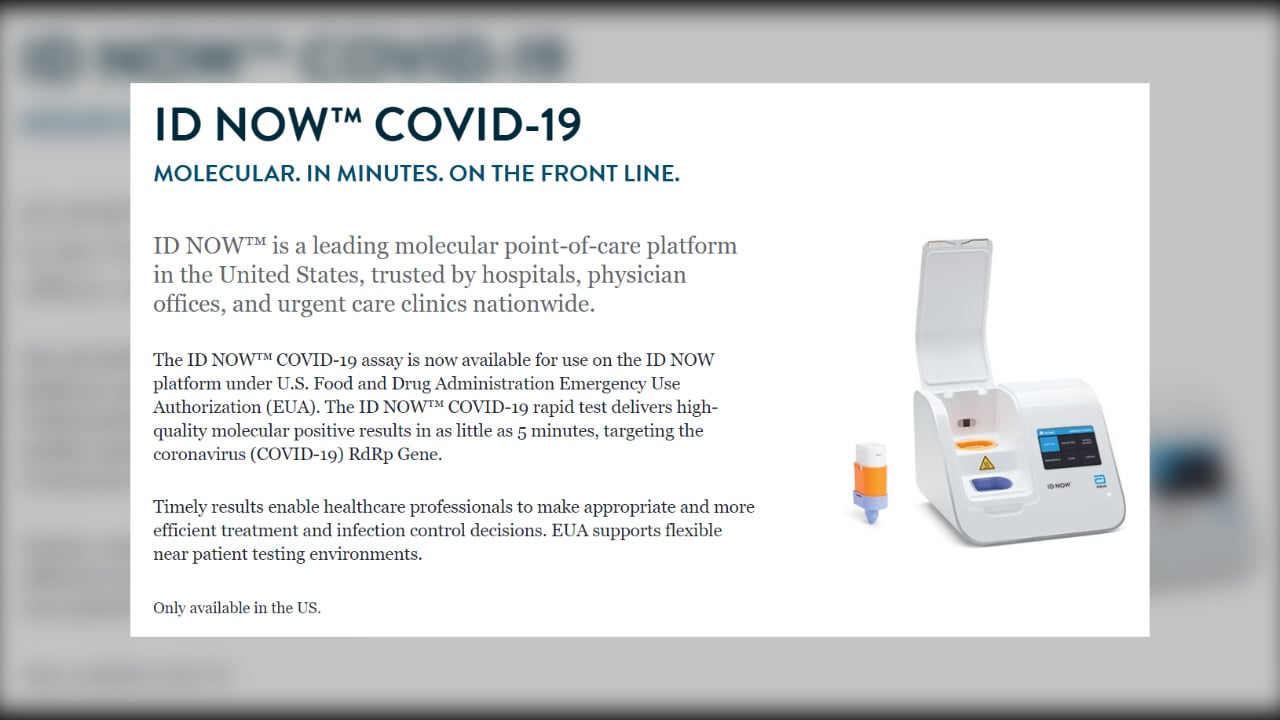
Id Now Covid 19 Assay
The id now covid 19 assay is now available for use on the id now platform under us.

Id now covid 19 assay. It is comprised of a sample. Find out more about this innovative technology and its impact here. Taking covid 19 testing to a new level the revolutionary navica app helps people navigate daily life in a new normal.
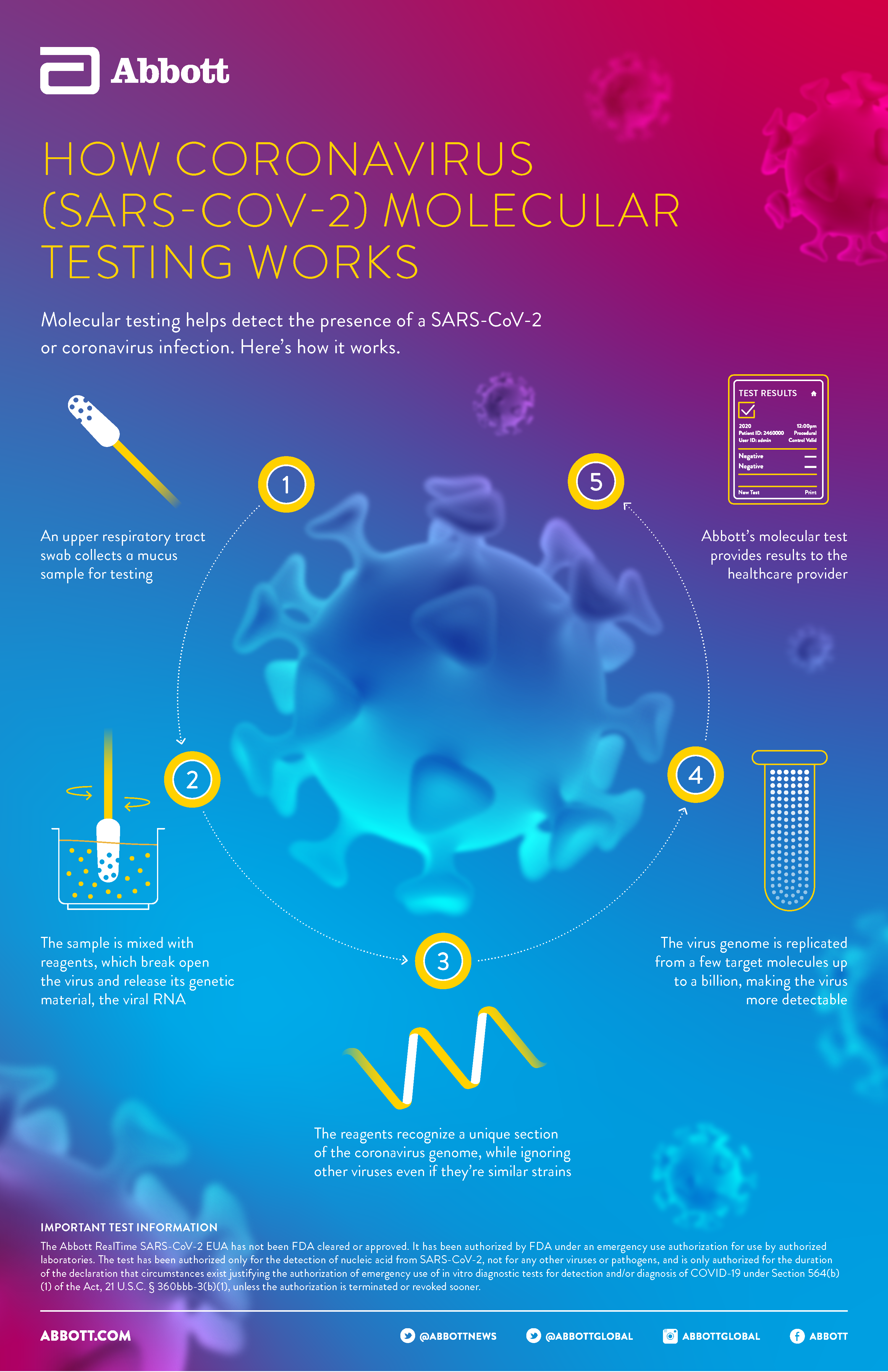
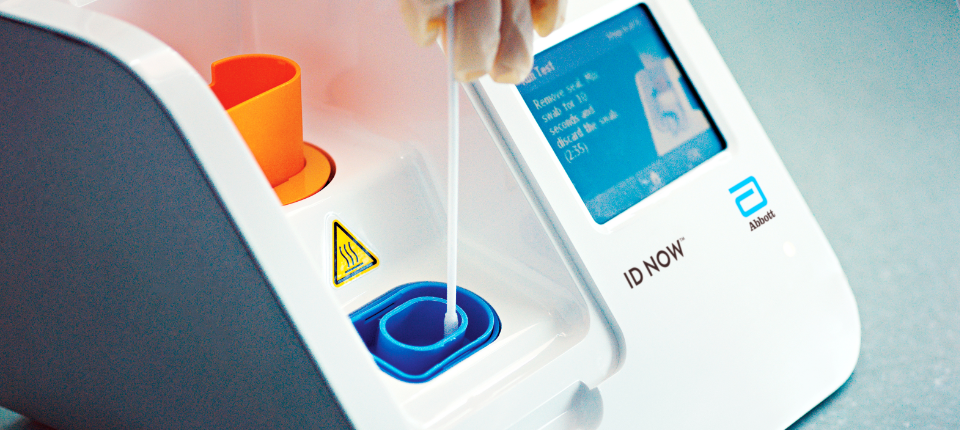
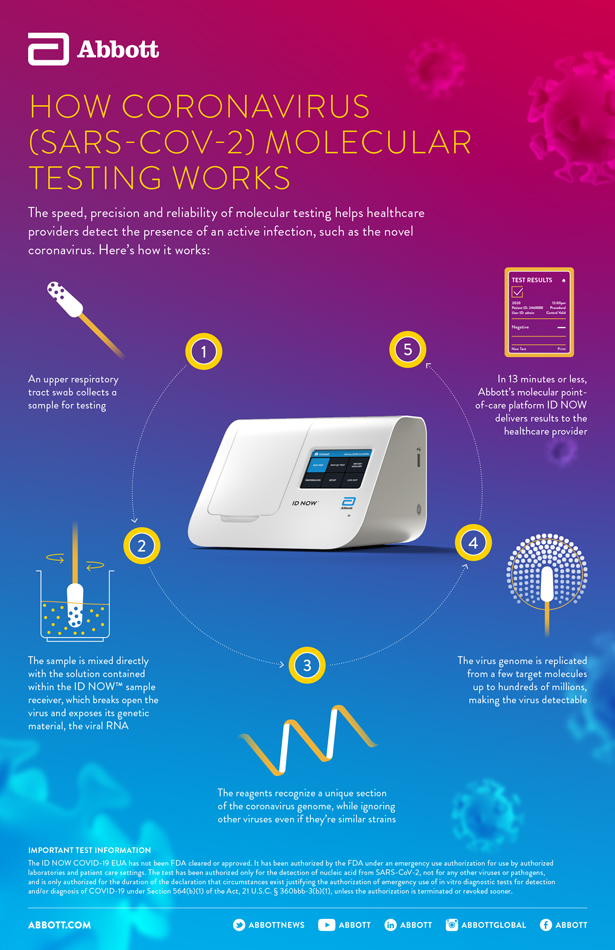
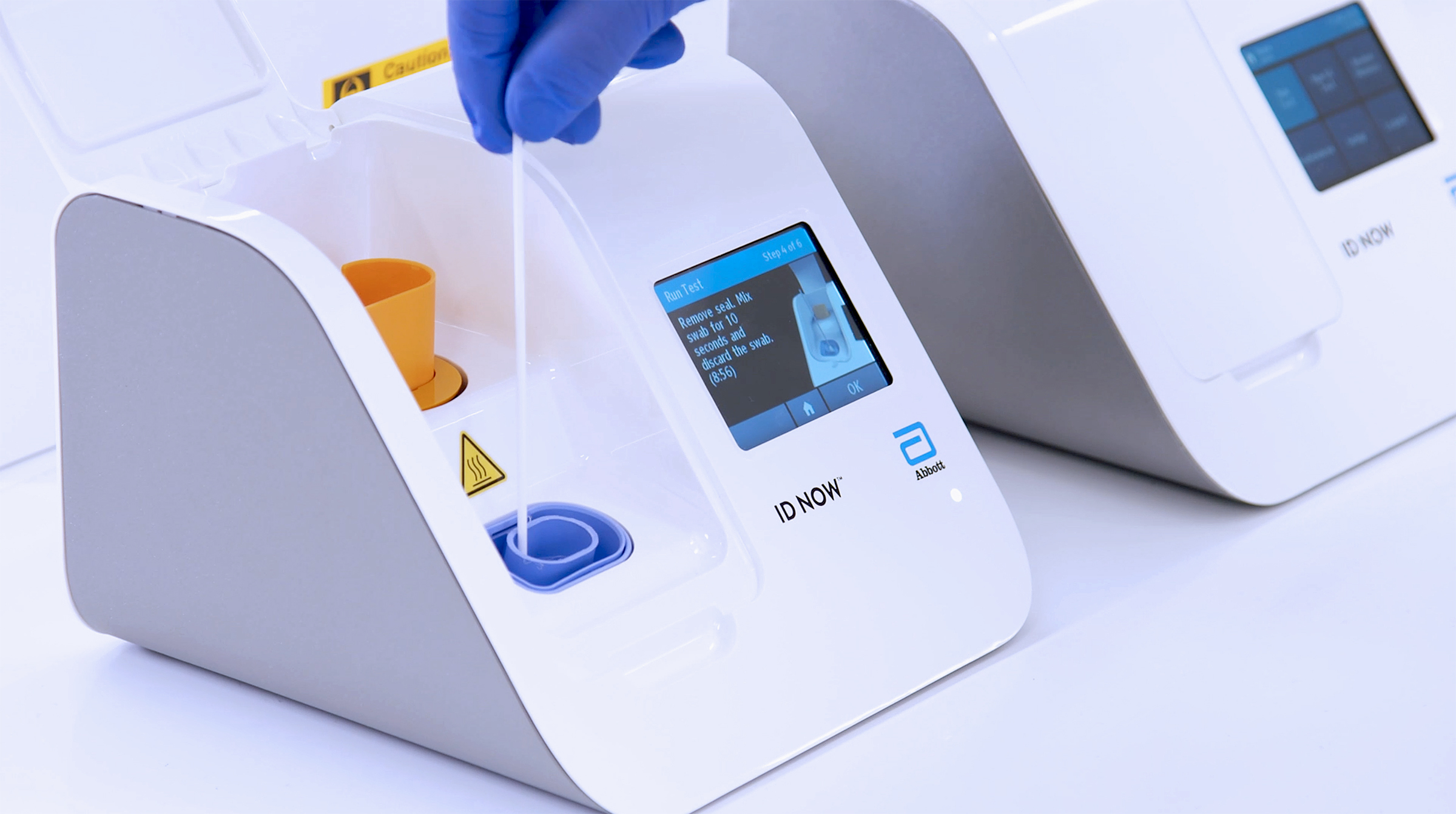
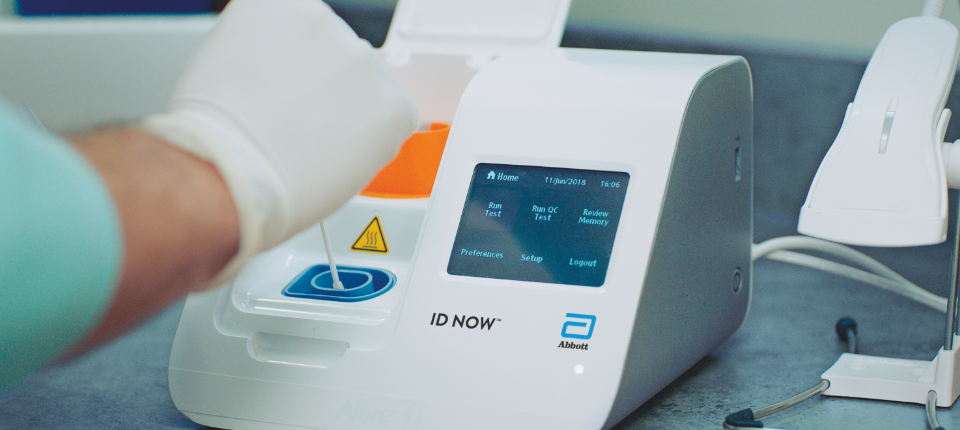
Id now covid 19 assay performed on the id now instrument is a rapid molecular in vitro diagnostic test utilizing an isothermal nucleic acid amplification technology intended for the qualitative detection of nucleic acid from the sars cov 2 viral rna in direct nasal nasopharyngeal or throat swabs from individuals who are suspected of covid 19 by their healthcare provider. Many nations are employing combinations of containment and mitigation strategies where early diagnosis of covid 19 is vit. The new abbott id now covid 19 test runs on abbotts id now tm platform a lightweight box 66 pounds and the size of a small toaster that can sit in a variety of locations.
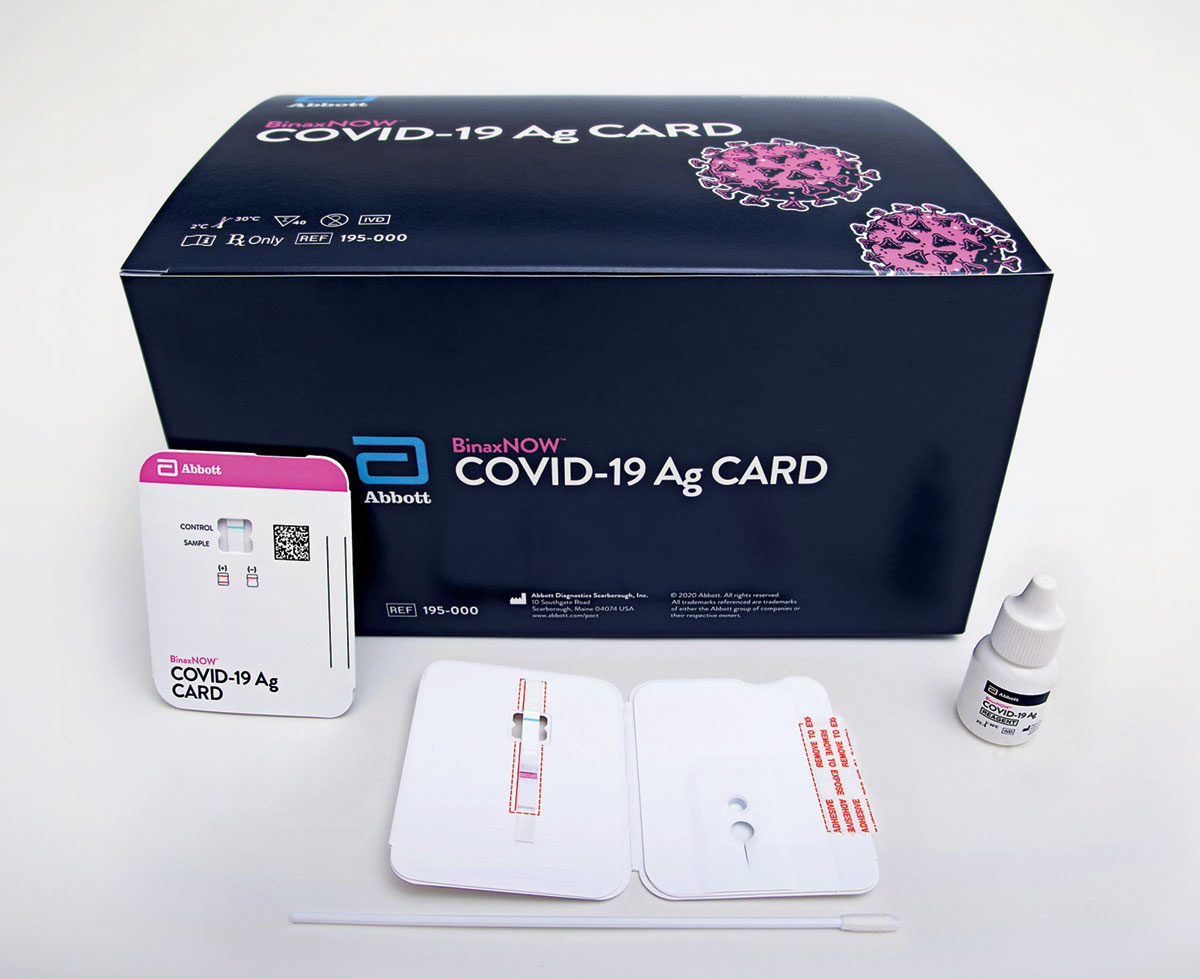
This sensitivity among other concerns should be taken into consideration when using this test for patients with a low suspicion for covid 19 disease. Navica displays results from the 15 minute abbott binaxnow covid 19 ag card rapid antigen test to help individuals make informed decisions. The id now covid 19 rapid test delivers high quality molecular positive results in as little as 5 minutes targeting the coronavirus covid 19 rdrp gene.
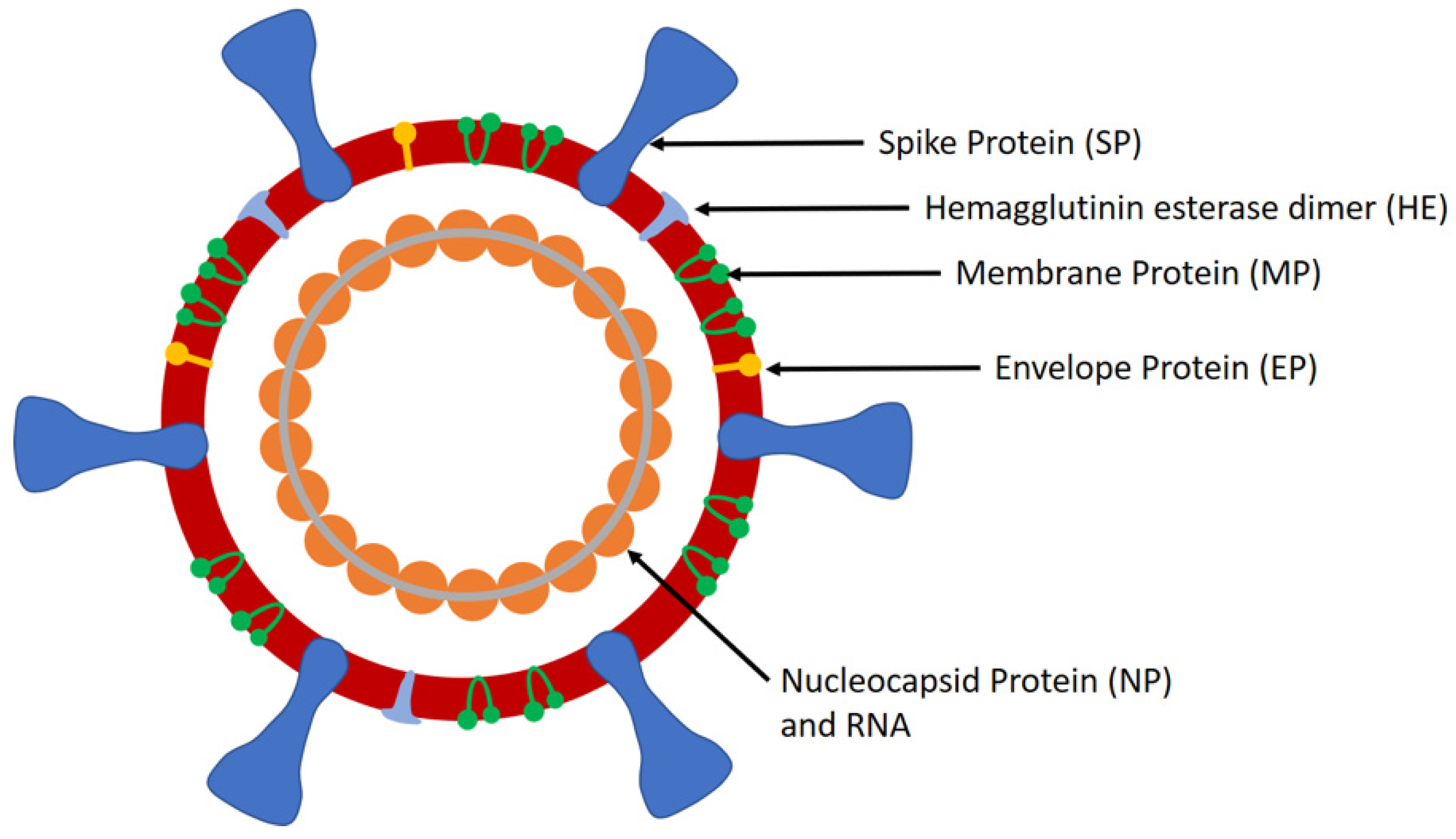
Id now performs well for strong and moderately positive samples but has reduced sensitivity for weakly positive samples. Id now covid 19 is an automated assay that utilizes isothermal nucleic acid amplification technology for the qualitative detection of sars cov 2 viral nucleic acids. In parallel id now has demonstrated 947 positive agreement and 986 negative agreement compared to the centers for disease control cdc 2019 novel coronavirus covid 19 real time rt pcr diagnostic panel.
Recently a novel coronavirus sars cov 2. The portable rapid molecular id now covid 19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as physicians offices urgent care clinics and other point of care locations. Coronavirus disease 2019 covid 19 has emerged rapidly spreading and severely straining the capacity of the global health community.

Covid 19 Diagnostic Tests Production Gaps Bioprocess Internationalbioprocess International
bioprocessintl.com

Abbott Releases Interim Clinical Study Data On Id Now Covid 19 Rapid Test Showing Strong Agreement To Lab Based Molecular Pcr Tests Pharmiweb Com
www.pharmiweb.com

States Finally Have High Speed Machines To Detect Covid 19 But Few Tests To Run On Them Cnnpolitics
www.cnn.com






















































/cdn.vox-cdn.com/uploads/chorus_asset/file/21822703/GettyImages_1269132621.jpg)