Test Rapido Covid 19 Lungene
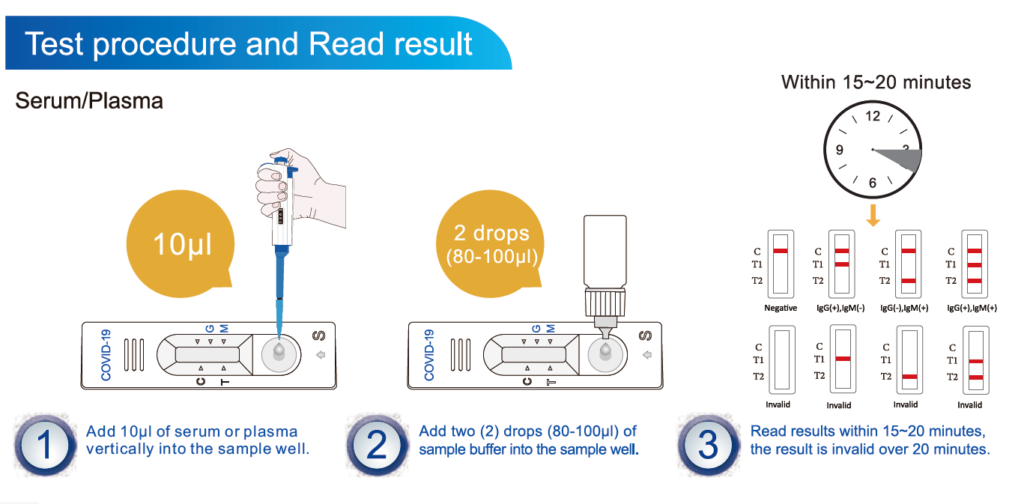
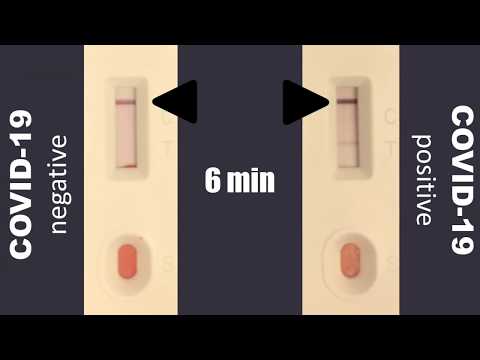
An igg line and an igm line.

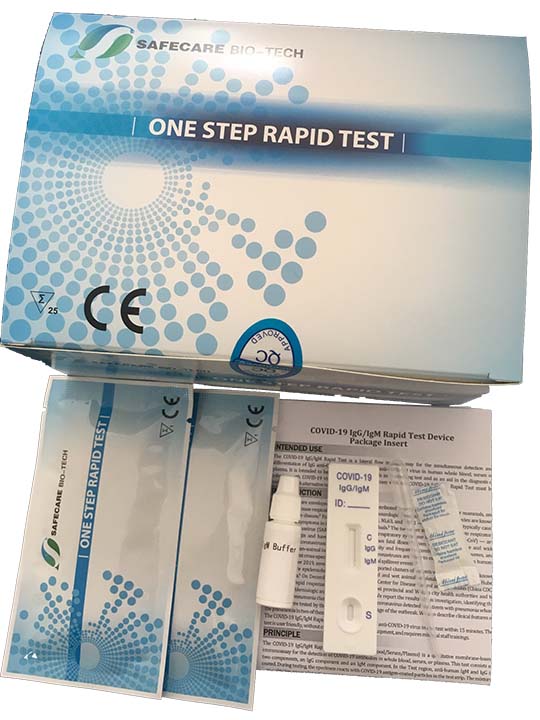
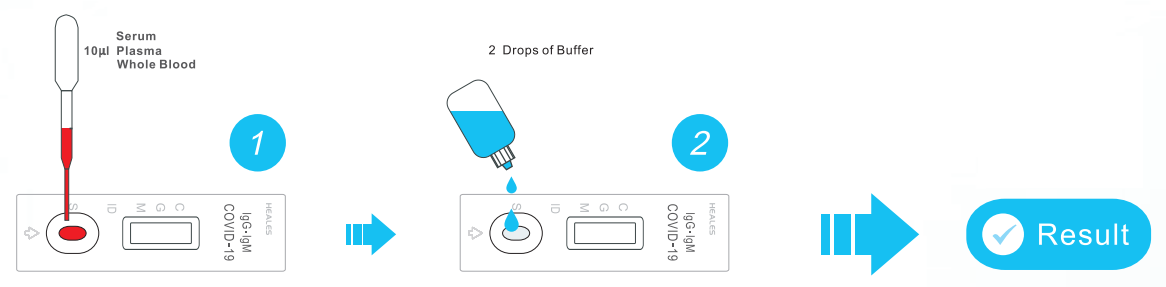
Test rapido covid 19 lungene. Buy reopentesti buy lungeneii. The nadal covid 19 iggigm rapid test is a chromatographic lateral flow immunoassay for the qualitative detection of anti sars cov 2 igg and igm in human whole blood serum or plasma samples. The covid 19 igmigg antibody rapid test is a qualitative test for covid 19 igm and igg antibodies to.
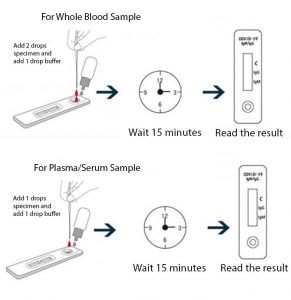
It is less complex and can provide results in less than 15 minutes. If playback doesnt begin shortly try restarting your device. Sars cov 2 covid 19 igmigg rapid test.
However it should not be used as the sole basis to diagnose or exclude sars cov 2 infection or to inform infection status is this product fda approved. Video con instrucciones de uso del test rapido covid 19 lungene. During an immune response to infection igm antibodies appear in the early stage followed by the emergence of igg during the mid to late disease stages.
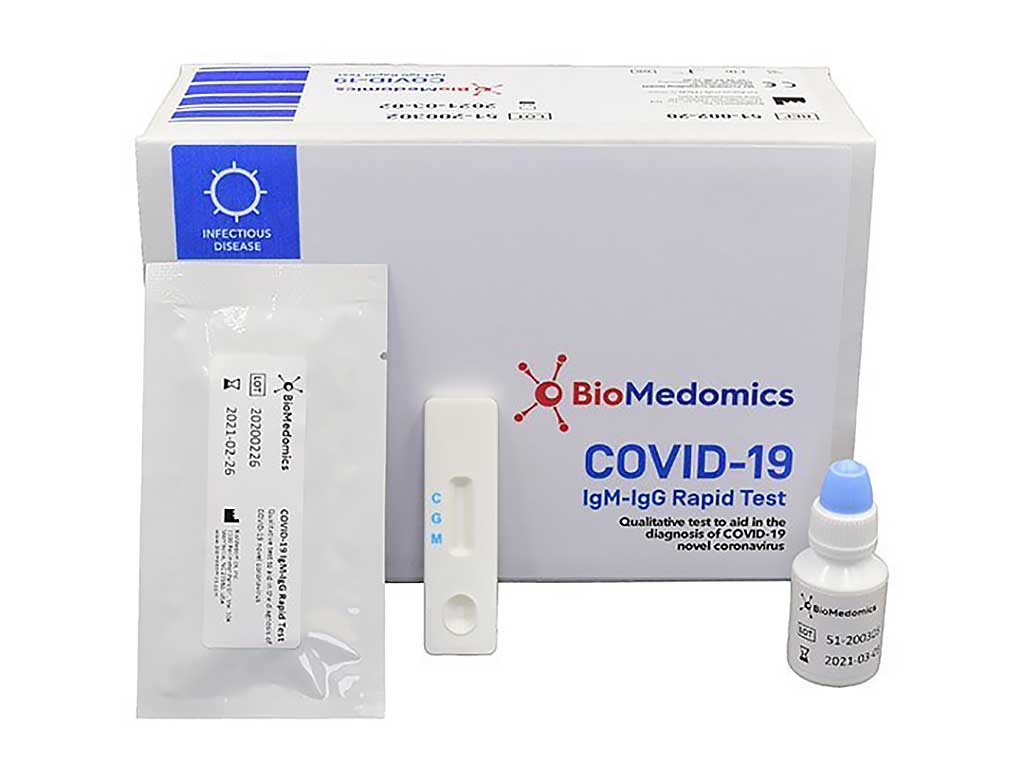
Federal authorities have granted emergency approval for a rapid covid 19 test which is reportedly highly accurate less invasive and can provide results in about 15 minutes. Covid 19 igmigg rapid test biomedomics has developed and launched one of the worlds best rapid point of care lateral flow immunoassays to aid in the diagnosis of coronavirus infection. The test detects both early marker and late marker igmigg antibodies in human finger prick capillary or venous whole blood serum and plasma samples.
How to using rapid test covid 19 lungene. Is a rapid chromatographic immunoassay for the qualitative detection of igg and igm antibodies against sars cov2 in human whole blood serum or plasma. El kit de diagnostico lungene iggigm de covid 19 debe ser utuilizado por trabajadores de la.
Food and drug administration fda for the fastest available molecular point of care test for the detection of novel coronavirus covid 19 delivering positive results in as little as five minutes and negative results in 13 minutes. This test contains two test lines. The covid 19 iggigm whole bloodserumplasma rapid test is a lateral flow immunoassay intended for the qualitative detection and differentiation of igm and igg antibodies to sars cov 2 in human venous whole blood plasma from anticoagulated blood li heparin k2edta and sodium citrate or serum.
It is important to note that in the early stages of the infection anti sars cov 2 igg and igm may be below the detection limit of the test.




















































.jpg)